12 14, 2021
Thalamic nucleogenesis
The ontogenetic organization of nuclear brain structure
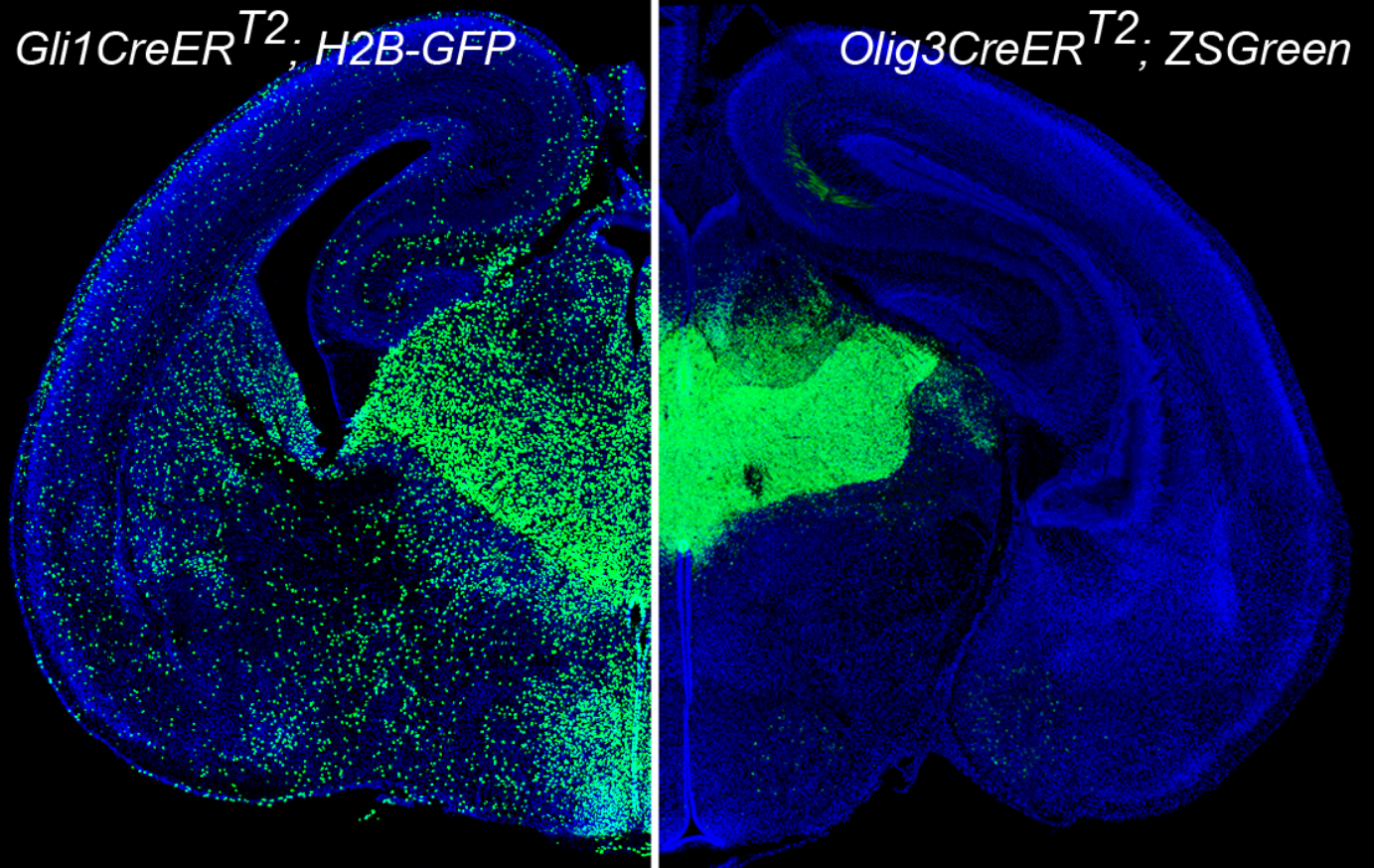
Genetic labeling of neural progenitors in thalamus
The structure of neural systems in the developing mammalian brain is dictated by tightly regulated cell generation and migration patterns. These include layered structures like the neocortex and cerebellum, and nuclear structures such as the thalamus and hypothalamus. Although the organization of layered structures has been well-studied, much less is known about how nuclear structures in the brain are formed. The thalamus is composed of dozens of spatially complex nuclei and plays a crucial role in regulating the transmission of sensory and motor signals to the cerebral cortex. Previous studies using population-level genetic fate mapping have revealed that thalamic nuclei are derived from two progenitor domains pTH-R and pTH-C, but how thalamic progenitors produce neurons and contribute to individual nuclei remains unclear.
In this study, Dr. Qing-Feng Wu’s research group at the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, in collaboration with the University of Minnesota and the University of Pennsylvania, used an in vivo clonal analysis approach to study the formation of thalamic nuclei. Three different inducible CreER driver mouse lines were combined with MADM (Mosaic Analysis with Double Markers) reporters, which allows for lineage tracing of neural progenitors in the thalamus at the clonal level. The findings reported herein reveal the remarkable coupling of patterning, cell division and cell fate specification in a spatiotemporally specific manner to form distinct thalamic nuclei.
The following are the main research findings in this study. First, clonal analysis of thalamic neural progenitors revealed a transition of cell division mode from symmetric proliferative division to asymmetric neurogenesis division to symmetric neurogenic division, similar to the neocortex. Second, each clone produced from individual thalamic progenitors contributed to multiple nuclei instead of a single nucleus, whereas symmetric clones populated more nuclei on average than asymmetric clones. Third, clones produced by neural progenitor cells with similar spatial positions were distributed in distinct subsets of nuclei. Furthermore, given that functionally distinct nuclei do not completely segregate in origin, the findings suggest that the spatial information encoded by neural progenitor cells could be the key factor in determining the formation of nuclei. Lastly, lineage tracing of individual intermediate progenitor cells showed that most of the labeled clones contained 4 or fewer cells of neuronal morphology, and a considerable number of individual clones generated neurons populating 2 or more nuclei. Importantly, these results indicate that even the last division of such progenitor cells can generate cells in multiple distinct nuclei, suggesting that there are prolonged mechanisms of nuclear fate specification in the thalamus during development. Taken together, this study has established a framework for decoding the organizational principles underlying the formation of nuclear structures in the mammalian brain.
The study was published online in PLOS Biology on April 23, 2018.(http://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.2005211). The article is titled In Vivo Clonal Analysis Reveals Spatiotemporal Regulation of Thalamic Nucleogenesis.
This study was supported by the National Natural Science Foundation of China, the Chinese Academy of Sciences Pilot Project A, and the State Key Laboratory of Molecular Developmental Biology.